
The questionable contribution of the Neolithic and the Bronze Age to European craniofacial form
Abstract
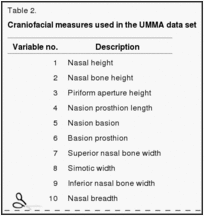
Many human craniofacial dimensions are largely of neutral adaptive significance, and an analysis of their variation can serve as an indication of the extent to which any given population is genetically related to or differs from any other. When 24 craniofacial measurements of a series of human populations are used to generate neighbor-joining dendrograms, it is no surprise that all modern European groups, ranging all of the way from Scandinavia to eastern Europe and throughout the Mediterranean to the Middle East, show that they are closely related to each other. The surprise is that the Neolithic peoples of Europe and their Bronze Age successors are not closely related to the modern inhabitants, although the prehistoric/modern ties are somewhat more apparent in southern Europe. It is a further surprise that the Epipalaeolithic Natufian of Israel from whom the Neolithic realm was assumed to arise has a clear link to Sub-Saharan Africa. Basques and Canary Islanders are clearly associated with modern Europeans. When canonical variates are plotted, neither sample ties in with Cro-Magnon as was once suggested. The data treated here support the idea that the Neolithic moved out of the Near East into the circum-Mediterranean areas and Europe by a process of demic diffusion but that subsequently the in situ residents of those areas, derived from the Late Pleistocene inhabitants, absorbed both the agricultural life way and the people who had brought it.
Among those who deal with the background of European history, there is a generally accepted view that the foraging way of life in the post-Pleistocene Mesolithic was succeeded by the Neolithic farming way of life. With the addition of metallurgy, the Neolithic morphed into the Bronze Age, which was succeeded by the Iron Age and the more recent European civilization (1–4). Further there is a general acceptance of the assumption that the farming way of life of the Neolithic arose in the Middle East ≈11,000 years ago and spread to the western edge of Europe by about 6,500 years ago (5–10). Researchers have questioned whether that spread took place by cultural diffusion to in situ people (11) or whether it was a “wave of advance” or a matter of “demic diffusion,” the actual movement of groups of people (see refs. 1, 8, and 12–15). Some researchers have observed that, although the two possible modes of Neolithic spread need not be mutually exclusive (see refs. 9 and 12), principal components analysis of allele frequencies in living humans shows a southeast–northwest cline that favors the idea that the spread had been the result of actual demic movement rather than by diffusion of cultural elements to preexisting populations (see refs. 11–15).
Previous assessments of the Neolithic spread from the Middle East westward have been based on a consideration of tools and pottery on the one hand and genetically controlled aspects of living human populations on the other (14, 15). Here we offer an assessment based on a comparison of a set of metric dimensions of both prehistoric and more recent human craniofacial morphology. Craniofacial analysis has been previously applied to this question, but the comparison to living populations was not done (16). It has already been shown that the quantitative treatment of craniofacial form produces a picture of the movement of human populations from Asia into the New World that is largely compatible with the picture produced by the molecular genetic comparison of nucleotide haplotypes (17, 18).
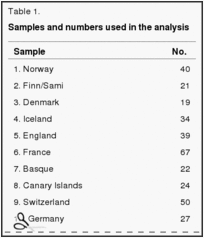
The underlying reason that such different approaches yield comparable results is that neither the nucleic acid components identified nor the particular craniofacial dimensions used have any obvious adaptive value. Both evidently behave in a manner compatible with what has been called the “neutral theory,” where the traits assessed are under genetic control and the differences between groups are principally the result of genetic drift (12–22). What they show, then, is the extent of genetically shared relationships between adjacent populations. Here we offer a comparable treatment of samples of recent and prehistoric human populations running from the Middle East to the western edge of the Eurasian continent, north to Crimea, east to Mongolia, and southward through Nubia and Somalia plus samples from North Africa and representatives of the Niger-Congo-speaking peoples of Sub-Saharan Africa (Table 1). Teeth and the tooth-bearing parts of facial skeletons of course do reflect differences in response to the forces of selection on different populations (23), but they were left out of our analysis.
Neighbor-Joining Comparisons
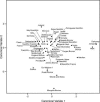
A battery of 24 craniofacial measurements (Table 2) was used to compare the similarities and differences of living human populations and their prehistoric predecessors where possible throughout the area in question. The significance of the difference between any pair of the total sample can be assessed from Mahalanobis D2 figures (24), and a graphic depiction of the similarities and distinctions of the various groups tested can be seen from the dendrogram produced by using the D2 figures as input for the neighbor-joining procedure (Fig. 1) (25). To compute the Mahalanobis distances, we used a pooled within-group covariance matrix derived from all groups and weighted by sex and group sample size. The neighbor-joining method can be used for discrete differences, as is done with molecular data, or it can be used on continuous data, as we have done here (25). Assessments can also be made with canonical variate plots, which have the added advantage that single individuals can be placed in relation to the other samples used (Fig. 2) (29–32).
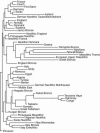
It is no surprise to discover that individual samples of recent humans tie more closely with other samples of extant people from the same part of the world than with more distant peoples. What does come as a surprise is that the Neolithic samples tend to tie with Neolithic samples across the entire range from east to west but do not cluster with the living people in many of the areas tested. There is more of a link between the prehistoric and modern samples in southern Europe as opposed to the picture in central and northern Europe. Much the same is true for the Bronze Age samples, although these do tend to tie to the preceding Neolithic in the same part of the range tested.
Unlike the Neolithic, Bronze Age, and modern samples, the Palaeolithic samples are not from single sites. There is no single European Upper Palaeolithic sample large enough to run as a single twig in a dendrogram. Instead, we had to use Cro-Magnon 1, La Ronde du Barry, Abri Pataud, Saint Germain-La Rivière, and Le Placard, all from southwestern France, plus Obercassel 1 from western Germany, and Předmostí 3 and 4 from the Czech Republic. Measurements of the latter two specimens were taken on casts because the originals had been destroyed by retreating Germans near the end of World War II (33). The same kind of problem of finding more than one individual in a burial site also tended to be true for some of the available Mesolithic of Europe. Individual specimens from Brittany to Monaco (Gramat, Rastel, Recheril and Téviec) were lumped together to make the European Mesolithic sample. There are larger Mesolithic samples, but we were not able to get permission to work on them. The North African Epipalaeolithic sample was made on the basis of specimens from Afalou in Algeria and Taforalt in Morocco. The Natufian sample from Israel is also problematic because it is so small, being constituted of three males and one female from the Late Pleistocene Epipalaeolithic (34) of Israel, and there was no usable Neolithic sample for the Near East.
The difficulty in making comparisons with Neolithic and Palaeolithic samples is the result of the very different treatment of the deceased. Neolithic communities established cemeteries where the remains of the departed accumulated in some numbers. Most Upper Palaeolithic peoples tended to bury the dead singly and in widely separated locations. Furthermore, Neolithic pottery became fractured with considerable frequency, leaving potsherds in quantity at Neolithic sites. Consequently there may well have been a tendency to overestimate the size of Neolithic populations vis-à-vis the contemporary surviving foragers (6, 35, 36). Despite the small numbers and scattered locations of the Late Pleistocene specimens, they tend to cluster with each other rather than with any groups of more recent date.
In dendrograms such as Fig. 1, the little Natufian sample clusters with the Mesolithic of France, the North African Epipalaeolithic, and the European Upper Palaeolithic, but the lengths of each of these twigs show that the relationships are comparatively remote. These are all Late Pleistocene or very early post-Pleistocene groups, and they are also noticeably more robust than more recent human groups. The three Niger-Congo-speaking groups (the Congo from Gabon, the Dahomey from Benin, and the Haya from Tanzania) cluster together away from most of the other samples. They do show a somewhat more distant link to the Nubians and the Nubian Bronze Age, who are so close to each other that they were combined for subsequent analyses.
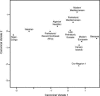
When the samples used in Fig. 1 are compared by the use of canonical variate plots as in Fig. 2, the separateness of the Niger-Congo speakers is again quite clear. Interestingly enough, however, the small Natufian sample falls between the Niger-Congo group and the other samples used. Fig. 2 shows the plot produced by the first two canonical variates, but the same thing happens when canonical variates 1 and 3 (not shown here) are used. This placement suggests that there may have been a Sub-Saharan African element in the make-up of the Natufians (the putative ancestors of the subsequent Neolithic), although in this particular test there is no such evident presence in the North African or Egyptian samples. As shown in Fig. 1, the Somalis and the Egyptian Bronze Age sample from Naqada may also have a hint of a Sub-Saharan African component. That was not borne out in the canonical variate plot (Fig. 2), and there was no evidence of such an involvement in the Algerian Neolithic (Gambetta) sample.
Combining Samples
When groups that are close to each other in the dendrogram in Fig. 1 are combined to make a single dendrogram twig, the picture is simplified, but much the same conclusion is supported. Czech, Denmark, England, Etruscan, Finn/Sami, France, Germany, Iceland, Norway, Sardinia, and Swiss samples are combined to make a sample designated as “Modern Europe.” Algeria, Berber, Greece, Iran/Iraq, Italy, Morocco, Sicily, and Tunisia samples were combined to generate a “Modern Mediterranean” twig, and the Algerian Neolithic was run as a separate twig. Next the Congo, Dahomey, and Haya samples were run as a “Niger-Congo” twig. Then Neolithic samples from Denmark, England, France, Germany, and Portugal were combined with Bronze Age samples from England, Jericho, and Mongolia to make a “Late Prehistoric Eurasia” sample. Mongolia is a long way east of any of the other samples used, but it has previously been shown that the Mongolian Bronze Age sample is unrelated to modern Mongols and has more in common with prehistoric Europeans and the Native Americans of the United States–Canada border (17).
Next the Portuguese Mesolithic, Greek Neolithic, Italy Eneolithic, and Swiss Neolithic samples and the Italian and Greek Bronze Age samples were combined to make a “Prehistoric Mediterranean” twig. Then Naqada Bronze Age Egyptian, the Nubian, Nubia Bronze Age, Israeli Fellaheen (Arabic farmers), and Somali samples were lumped as “Prehistoric/Recent Northeast Africa.” The Natufians and the Algerian Neolithic samples were run as separate twigs, and there were separate twigs for Basques and Canary Islanders. Figure 3 shows the results of running all of these twigs in a single neighbor-joining dendrogram. Only 18 of the 24 variables were used to construct Fig. 3, allowing us to add the Basque sample. When the Basques are left out and all 24 variables are used, the main twigs in the resulting dendrogram relate to each other in exactly the same way as those in the 18-variable version shown in Fig. 3. The D2 figures that were used in the construction of Fig. 3 are printed in Table 3.
There are some generalizations that are apparent from the picture presented in both the greater individual numbers of twigs shown in Fig. 1 and the combined pattern shown in Fig. 3. When the maximum number of twigs is plotted, despite the very small numbers involved, the Late Pleistocene samples from Israel, Europe, and North Aftica tend to link to each other before they tie to the modern representatives of each of the areas in question, as shown in Fig. 1. In that run, the Natufian of Israel ties to the French Mesolithic and then to the Afalou/Taforalt sample from North Africa. These then link with the European Upper Palaeolithic sample and, somewhat surprisingly, with the Chandman (the Mongolian Bronze Age sample) and finally, at the next step, with the Danish Neolithic. One of the things that these geographically diverse groups clearly have in common is a degree of robustness that sets them apart from the recent inhabitants of the areas in which they are found.
Apart from the quantitative relationships shown in Figs. Figs.1,1, ,2,2, ,3,3, ,4,4, most of the Neolithic samples in Europe share nonmetric features of the lateral edge of the orbit, the shape of the gonial angle of the mandible, and the configuration of menton that are present even when degrees of size and robustness vary between the regions represented. These nonmetric attributes all support the view that most of the Neolithic inhabitants of Europe tie more closely together with each other than with the living representatives of the areas in question. The principal exception to this generalization is one of the two small samples of the German Neolithic, the Mühlhausen sample, which ties closer metrically to the living inhabitants of the Middle East and North Africa. Metrically the other German Neolithic sample, Tauberbischofsheim, links with the living Central European samples. Nonmetrically, those two small German Neolithic samples also appear strikingly different from each other.
The Niger-Congo speakers (Congo, Dahomey, and Haya) cluster closely with each other and a bit less closely with the Nubian sample (both the recent and the Bronze Age Nubians) and more remotely with the Naqada Bronze Age sample of Egypt, the modern Somalis, and the Arabic-speaking Fellaheen (farmers) of Israel. When those samples are separated and run in a single analysis as in Fig. 1, there clearly is a tie between them that is diluted the farther one gets from Sub-Saharan Africa. The other obvious matter shown in Fig. 3 is the separate identity of the northern Europeans. This matter is treated in the next section.
Basques, Berbers, and Canary Islanders
When the number of variables is reduced from 24 to 18, the Basque sample can be compared with the others in the data set (Fig. 3). The Mahalanobis D2 figures for the samples used in Fig. 3 are shown in Table 3.
The Basque language is a linguistic isolate unrelated to any other language (37), and there is a long-held idea that the Basques may represent a modern survival of the Pleistocene human inhabitants of western Europe (38). Our measurements were made on the sample gathered from the French side of the French/Spanish frontier that runs through Basque country in southwestern France. These specimens were stored in the Broca collection at the Musée de l'Homme in Paris. Paul Broca himself had promoted the view that the Basques represent the continuing existence of the kind of Upper Paleolithic population excavated at the Cro-Magnon rock shelter in the village of Les Eyzies in the Dordogne region of southwestern France in 1868 (38–41). Shortly thereafter the “old man” (“le vieillard”) found in that rock shelter was elevated to the status of typifying a whole “Cro-Magnon race” regarded as ancestral to not only the Basques but also the aboriginal inhabitants of the Canary Islands (38, 42–45).
When the Basques are run with the other samples used in Fig. 1, they link with Germany and more remotely with the Canary Islands. They are clearly European, although the length of their twig indicates that they have a distinction all their own. It is clear, however, that they do not represent a survival of the kind of craniofacial form indicated by Cro-Magnon any more than do the Canary Islanders, nor does either sample tie in with the Berbers of North Africa as has previously been claimed (38, 45–46). This is particularly well documented when the 18 variables are used to generate a plot of the first two canonical variates as shown in Fig. 4. In this figure, one can see a clear link between the Niger-Congo sample and the Natufians. The Prehistoric/Recent Northeast African sample also has a subsequent link to the Niger-Congo sample in Fig. 3. Yet the D2 values in Table 3 show that it is slightly closer to Late Prehistoric Eurasia than to the Algerian Neolithic, Modern Europe, and Modern Mediterranean and that it is farthest from the Niger-Congo, the Natufians, and the Basques. Although the Algerian Neolithic sample has an even more residual link to this cluster, the D2 figures in Table 3 show that it is almost as far from the Niger-Congo twig as from the Basques and Natufians. The generally high D2 values for the Natufian sample in Table 3 are almost certainly a reflection of the very small sample size.
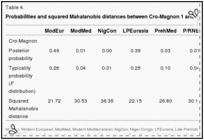
To test the analysis shown in Fig. 3, Cro-Magnon (Fig. 4, ×) was removed from the European Upper Palaeolithic sample and run as a single individual. Interestingly enough, Cro-Magnon is not close to any more recent sample. Clearly, Cro-Magnon is not the same as the Basque or Canary Island samples. Fig. 4 plots the first and second canonical variates against each other, but that conclusion is even more strongly supported when canonical variate 3 (not shown here) is plotted with variate 1. The probabilities of Cro-Magnon's ties to any of the groups in Figs. Figs.33 and and44 are shown in Table 4. If this analysis shows nothing else, it demonstrates that the oft-repeated European feeling that the Cro-Magnons are “us” (47) is more a product of anthropological folklore than the result of the metric data available from the skeletal remains.
Conclusions
The assessment of prehistoric and recent human craniofacial dimensions supports the picture documented by genetics that the extension of Neolithic agriculture from the Near East westward to Europe and across North Africa was accomplished by a process of demic diffusion (11–15). If the Late Pleistocene Natufian sample from Israel is the source from which that Neolithic spread was derived, then there was clearly a SubSaharan African element present of almost equal importance as the Late Prehistoric Eurasian element. At the same time, the failure of the Neolithic and Bronze Age samples in central and northern Europe to tie to the modern inhabitants supports the suggestion that, while a farming mode of subsistence was spread westward and also north to Crimea and east to Mongolia by actual movement of communities of farmers, the indigenous foragers in each of those areas ultimately absorbed both the agricultural subsistence strategy and also the people who had brought it. The interbreeding of the incoming Neolithic people with the in situ foragers diluted the Sub-Saharan traces that may have come with the Neolithic spread so that no discoverable element of that remained. This picture of a mixture between the incoming farmers and the in situ foragers had originally been supported by the archaeological record alone (6, 9, 33, 34, 48, 49), but this view is now reinforced by the analysis of the skeletal morphology of the people of those areas where prehistoric and recent remains can be metrically compared.
Notes
Author contributions: C.L.B. designed research; C.L.B., N.S., C.B.Q., S.C.F., A.R.N., S.K.M., and Q.P. performed research; C.L.B. and N.S. analyzed data; and C.L.B. wrote the paper.
Conflict of interest statement: No conflicts declared.
References
Formats:
- Article |
- PubReader |
- ePub (beta) |
- PDF (355K) |
- Citation
-
Old World sources of the first New World human inhabitants: a comparative craniofacial view.[Proc Natl Acad Sci U S A. 2001]Brace CL, Nelson AR, Seguchi N, Oe H, Sering L, Qifeng P, Yongyi L, Tumen D. Proc Natl Acad Sci U S A. 2001 Aug 14; 98(17):10017-22. Epub 2001 Jul 31.
-
Population continuity, demic diffusion and Neolithic origins in central-southern Germany: the evidence from body proportions.[Homo. 2009]Gallagher A, Gunther MM, Bruchhaus H. Homo. 2009; 60(2):95-126. Epub 2009 Mar 4.
-
Iberia: population genetics, anthropology, and linguistics.[Hum Biol. 1999]Arnaiz-Villena A, Martínez-Laso J, Alonso-García J. Hum Biol. 1999 Oct; 71(5):725-43.
-
Origins and genetic legacy of Neolithic farmers and hunter-gatherers in Europe.[Science. 2012]Skoglund P, Malmström H, Raghavan M, Storå J, Hall P, Willerslev E, Gilbert MT, Götherström A, Jakobsson M. Science. 2012 Apr 27; 336(6080):466-9.
-
Human genomic diversity in Europe: a summary of recent research and prospects for the future.[Eur J Hum Genet. 1993]Cavalli-Sforza LL, Piazza A. Eur J Hum Genet. 1993; 1(1):3-18.
-
Genomic insights into the origin of farming in the ancient Near East[Nature. 2016]Lazaridis I, Nadel D, Rollefson G, Merrett DC, Rohland N, Mallick S, Fernandes D, Novak M, Gamarra B, Sirak K, Connell S, Stewardson K, Harney E, Fu Q, Gonzalez-Fortes G, Jones ER, Roodenberg SA, Lengyel G, Bocquentin F, Gasparian B, Monge JM, Gregg M, Eshed V, Mizrahi AS, Meiklejohn C, Gerritsen F, Bejenaru L, Blüher M, Campbell A, Cavalleri G, Comas D, Froguel P, Gilbert E, Kerr SM, Kovacs P, Krause J, McGettigan D, Merrigan M, Merriwether DA, O'Reilly S, Richards MB, Semino O, Shamoon-Pour M, Stefanescu G, Stumvoll M, Tönjes A, Torroni A, Wilson JF, Yengo L, Hovhannisyan NA, Patterson N, Pinhasi R, Reich D. Nature. 2016 Aug 25; 536(7617): 419-424
-
Ancient human genomes suggest three ancestral populations for present-day Europeans[Nature. 2014]Lazaridis I, Patterson N, Mittnik A, Renaud G, Mallick S, Kirsanow K, Sudmant PH, Schraiber JG, Castellano S, Lipson M, Berger B, Economou C, Bollongino R, Fu Q, Bos KI, Nordenfelt S, Li H, de Filippo C, Prüfer K, Sawyer S, Posth C, Haak W, Hallgren F, Fornander E, Rohland N, Delsate D, Francken M, Guinet JM, Wahl J, Ayodo G, Babiker HA, Bailliet G, Balanovska E, Balanovsky O, Barrantes R, Bedoya G, Ben-Ami H, Bene J, Berrada F, Bravi CM, Brisighelli F, Busby GB, Cali F, Churnosov M, Cole DE, Corach D, Damba L, van Driem G, Dryomov S, Dugoujon JM, Fedorova SA, Romero IG, Gubina M, Hammer M, Henn BM, Hervig T, Hodoglugil U, Jha AR, Karachanak-Yankova S, Khusainova R, Khusnutdinova E, Kittles R, Kivisild T, Klitz W, Kučinskas V, Kushniarevich A, Laredj L, Litvinov S, Loukidis T, Mahley RW, Melegh B, Metspalu E, Molina J, Mountain J, Näkkäläjärvi K, Nesheva D, Nyambo T, Osipova L, Parik J, Platonov F, Posukh O, Romano V, Rothhammer F, Rudan I, Ruizbakiev R, Sahakyan H, Sajantila A, Salas A, Starikovskaya EB, Tarekegn A, Toncheva D, Turdikulova S, Uktveryte I, Utevska O, Vasquez R, Villena M, Voevoda M, Winkler C, Yepiskoposyan L, Zalloua P, Zemunik T, Cooper A, Capelli C, Thomas MG, Ruiz-Linares A, Tishkoff SA, Singh L, Thangaraj K, Villems R, Comas D, Sukernik R, Metspalu M, Meyer M, Eichler EE, Burger J, Slatkin M, Pääbo S, Kelso J, Reich D, Krause J. Nature. 2014 Sep 18; 513(7518): 409-413
-
Early Back-to-Africa Migration into the Horn of Africa[PLoS Genetics. 2014]Hodgson JA, Mulligan CJ, Al-Meeri A, Raaum RL. PLoS Genetics. 2014 Jun 12; 10(6): e1004393
-
Detection of a population replacement at the Classic–Postclassic transition in Mexico[Proceedings of the Royal Socie...]González-José R, Martínez-Abadías N, González-Martín A, Bautista-Martínez J, Gómez-Valdés J, Quinto M, Hernández M. Proceedings of the Royal Society B: Biological Sciences. 2006 Dec 5; 274(1610): 681-688
-
The questionable contribution of the Neolithic and the Bronze Age to European cr...The questionable contribution of the Neolithic and the Bronze Age to European craniofacial formProceedings of the National Academy of Sciences of the United States of America. 2006 Jan 3; 103(1)242
Your browsing activity is empty.
Activity recording is turned off.
See more...-
Genetic evidence for the spread of agriculture in Europe by demic diffusion.[Nature. 1991]Sokal RR, Oden NL, Wilson CNature. 1991 May 9; 351(6322):143-5.
-
Old World sources of the first New World human inhabitants: a comparative craniofacial view.[Proc Natl Acad Sci U S A. 2001]Brace CL, Nelson AR, Seguchi N, Oe H, Sering L, Qifeng P, Yongyi L, Tumen DProc Natl Acad Sci U S A. 2001 Aug 14; 98(17):10017-22.
-
The neighbor-joining method: a new method for reconstructing phylogenetic trees.[Mol Biol Evol. 1987]Saitou N, Nei MMol Biol Evol. 1987 Jul; 4(4):406-25.
-
Old World sources of the first New World human inhabitants: a comparative craniofacial view.[Proc Natl Acad Sci U S A. 2001]Brace CL, Nelson AR, Seguchi N, Oe H, Sering L, Qifeng P, Yongyi L, Tumen DProc Natl Acad Sci U S A. 2001 Aug 14; 98(17):10017-22.
-
Review A nonracial craniofacial perspective on human variation: A(ustralia) to Z(uni).[Am J Phys Anthropol. 1990]Brace CL, Hunt KDAm J Phys Anthropol. 1990 Jul; 82(3):341-60.


 Facebook
Facebook
 Twitter
Twitter
 Google+
Google+